Controlled Products Regulations
SOR/88-66
Registration 1987-12-31
Regulations Respecting Controlled Products
P.C. 1987-2721 1987-12-31
Whereas the Minister of Consumer and Corporate Affairs has consulted with the government of each province and with such organizations representative of workers, organizations representative of employers and organizations representative of suppliers as the Minister deemed appropriate.
Therefore, Her Excellency the Governor General in Council, on the recommendation of the Minister of Consumer and Corporate Affairs, pursuant to subsection 11(2)Footnote *, sections 13Footnote * and 14Footnote * and subsection 15(1)Footnote * of the Hazardous Products Act, is pleased hereby to make the annexed Regulations respecting controlled products, effective October 31, 1988.
Return to footnote *S.C. 1987, c. 30, s. 1
Short Title
1 These Regulations may be cited as the Controlled Products Regulations.
Interpretation
2 (1) In these Regulations,
- Act
Act means the Hazardous Products Act; (Loi)
- CAS registry number
CAS registry number means the identification number assigned to a chemical substance by the Chemical Abstracts Service Division of the American Chemical Society; (numéro d’enregistrement CAS)
- complex mixture
complex mixture means a mixture that is a combination of many chemicals, has a commonly known generic name and is
(a) naturally occurring,
(b) a fraction of a naturally-occurring mixture that results from a separation process, or
(c) a modification of a naturally-occurring mixture or a modification of a fraction of a naturally-occurring mixture that results from a chemical modification process; (mélange complexe)
- hazard information
hazard information means information on the proper and safe storage, handling and use of a controlled product and includes information relating to its toxicological properties; (renseignements sur les dangers)
- LC50
LC50 means the concentration of a substance in air that, when administered by means of inhalation over a specified length of time in an animal assay, is expected to cause the death of 50 per cent of a defined animal population; (CL50)
- LD50
LD50 means the single dose of a substance that, when administered by a defined route in an animal assay, is expected to cause the death of 50 per cent of a defined animal population; (DL50)
- laboratory sample
laboratory sample means, in respect of a controlled product, a sample of the controlled product that is intended solely to be tested in a laboratory but does not include a controlled product that is to be used
(a) by the laboratory for testing other products, materials or substances, or
(b) for educational or demonstration purposes; (échantillon pour laboratoire)
- mixture
mixture means a combination of two or more products, materials or substances that do not undergo a chemical change as a result of interaction between them; (mélange)
- nurse
nurse means a registered nurse registered or licensed under the laws of a province; (infirmier ou infirmière)
- product identification number
product identification number means the number and letters, if any, specified in Column II of an item of List II in Schedule II to the Transportation of Dangerous Goods Regulations that correspond to the product specified in Column I of an item of that List; (numéro d’identification du produit)
- product identifier
product identifier means, in respect of a controlled product, the brand name, code name or code number specified by a supplier or the chemical name, common name, generic name or trade name; (identificateur du produit)
- research and development
research and development means systematic investigation or search carried out in a field of science or technology by means of experiment or analysis, other than investigation or search in respect of market research, sales promotion, quality control or routine testing of controlled products, and includes
(a) applied research, namely, work undertaken for the advancement of scientific knowledge with a specific practical application in view, and
(b) development, namely, use of the results of applied research for the purpose of creating new, or improving existing, processes or controlled products; (recherche et développement)
- risk phrase
risk phrase means, in respect of a controlled product or a class, division or subdivision of controlled products, a statement identifying a hazard that may arise from the nature of the controlled product or the class, division or subdivision of controlled products; (mention de risque)
- supplier identifier
supplier identifier means, in respect of a controlled product, the name of the supplier of the controlled product. (identificateur du fournisseur)
(2) For the purposes of Part II of the Act,
- bulk shipment
bulk shipment means a shipment of a controlled product that is contained, without intermediate containment or intermediate packaging, in
(a) a vessel with a water capacity of more than 454 litres,
(b) a freight container, a road vehicle, a railway vehicle, a portable tank, a freight container carried on a road vehicle, railway vehicle, ship or aircraft, or a portable tank carried on a road vehicle, railway vehicle, ship or aircraft,
(c) the hold of a ship, or
(d) a pipeline; (expédition en vrac)
- hazardous waste
hazardous waste means a controlled product that is intended for disposal or is sold for recycling or recovery; (résidu dangereux)
- work place
work place means a place where a person works for remuneration. (lieu de travail)
- SOR/97-543, s. 13(E)
Concentration Expressed as a Percentage
3 Where in these Regulations, other than in sections 11 and 36, the concentration of an ingredient is expressed as a percentage, the percentage shall be taken as an expression of the ratio of the weight of the ingredient to the weight of the controlled product.
PART IMaterial Safety Data Sheet
Exemptions
Concentration Cut-off
4 The sale or importation of a controlled product, other than a complex mixture or a component of a controlled product that is a complex mixture, is exempt from the application of paragraph 13(a) or 14(a) of the Act in respect of the requirement to disclose on a material safety data sheet the chemical identity and the concentration of
(a) an ingredient that is found in the controlled product in a concentration of less than 0.1 per cent and is a teratogen or an embryotoxin referred to in section 53, a carcinogen referred to in section 54, a reproductive toxin referred to in section 55, a respiratory tract sensitizer referred to in section 56 or a mutagen referred to in section 57; or
(b) an ingredient that is an ingredient other than an ingredient referred to in paragraph (a) and that is found in the controlled product in a concentration of less than one per cent, unless the ingredient is included in the Ingredient Disclosure List and the concentration specified for the ingredient in the List is 0.1 per cent.
Complex Mixtures
5 (1) The sale or importation of a controlled product that is a complex mixture is exempt from the application of paragraph 13(a) or 14(a) of the Act in respect of the requirement to disclose on a material safety data sheet the chemical identity and concentration of the ingredients of the complex mixture if the generic name of the complex mixture is disclosed on the material safety data sheet.
(2) The sale or importation of a controlled product that contains a component that is a complex mixture is exempt from the application of paragraph 13(a) or 14(a) of the Act in respect of the requirement to disclose on a material safety data sheet the chemical identity and concentration of the ingredients of the component if
(a) the component is found in the controlled product in a concentration of less than 0.1 per cent and is a teratogen or an embryotoxin referred to in section 53, a carcinogen referred to in section 54, a reproductive toxin referred to in section 55, a respiratory tract sensitizer referred to in section 56 or a mutagen referred to in section 57;
(b) the component is a component other than a component referred to in paragraph (a) and is found in the controlled product in a concentration of less than one per cent, unless the component is included in the Ingredient Disclosure List and the concentration specified for that component in the List is 0.1 per cent; or
(c) the commonly known generic name and concentration of the component in the controlled product is disclosed on the material safety data sheet.
Flavours and Fragrances
5.1 (1) For the purposes of this section,
- flavour
flavour means a product, material or substance that is used solely to impart a taste to another product, material or substance; (saveur)
- fragrance
fragrance means a product, material or substance that is used solely to impart a smell to another product, material or substance. (parfum)
(2) The sale or importation of a controlled product that is a flavour or fragrance is exempt, for as long as paragraph 12(b) of the Act is in force, from the application of paragraph 13(a) or 14(a) of the Act in respect of the requirement to disclose on a material safety data sheet the chemical identity and concentration of the ingredients of the controlled product if
(a) the generic chemical identities of the ingredients of the controlled product and the concentrations thereof are disclosed on the material safety data sheet;
(b) the supplier of the controlled product maintains a record of the chemical identities and concentrations of the ingredients of the controlled product at a place in Canada where an inspector may enter at any reasonable time for the purposes of the administration and enforcement of Parts II and III of the Act; and
(c) for the purposes of section 30, the supplier of the controlled product discloses on the material safety data sheet an emergency telephone number by means of which access to the information set out in the record referred to in paragraph (b) may be obtained at any time.
(3) The sale or importation of a controlled product that contains a component that is a flavour or fragrance is exempt, for as long as paragraph 12(b) of the Act is in force, from the application of paragraph 13(a) or 14(a) of the Act in respect of the requirement to disclose on a material safety data sheet the chemical identity and concentration of the ingredients of the component if
(a) the generic chemical identities of the ingredients of the component and the concentrations thereof are disclosed on the material safety data sheet;
(b) the supplier of the controlled product or of the component maintains a record of the chemical identities and concentrations of the ingredients of the component at a place in Canada where an inspector may enter at any reasonable time for the purposes of the administration and enforcement of Parts II and III of the Act; and
(c) the supplier of the controlled product or of the component discloses on the material safety data sheet, in parentheses after the information referred to in paragraph (a), the following information, namely,
(i) the product identifier of the component,
(ii) for the purposes of section 30, an emergency telephone number by means of which access to the information set out in the record referred to in paragraph (b) may be obtained at any time, and
(iii) a statement to the effect that in a medical emergency, a physician or nurse may obtain the chemical identity and concentration of any ingredient of the component set out in the record maintained pursuant to paragraph (b) by calling the emergency telephone number disclosed under subparagraph (ii) and specifying the product identifier of the component.
(4) Where an inspector obtains information from a record referred to in paragraph (2)(b) or (3)(b), the inspector shall keep the information confidential except for the purposes of the administration and enforcement of Parts II and III of the Act.
- SOR/89-150, s. 1
Controlled Products with Same Product Identifier
6 The sale or importation of a controlled product is exempt from the application of paragraph 13(a) or 14(a) of the Act in respect of the requirement to transmit, obtain or prepare a material safety data sheet for the controlled product if
(a) the controlled product is part of a shipment of controlled products that have the same product identifier and a material safety data sheet is transmitted with the shipment or is obtained or prepared for one of the controlled products; or
(b) the supplier has transmitted to the person to whom the controlled product is sold or the supplier who imports the controlled product has in his possession a material safety data sheet for a controlled product that has the same product identifier and the material safety data sheet
(i) discloses information that is current at the time of the sale or importation, and
(ii) was prepared and dated not more than three years before the date of the sale or importation.
Generic Material Safety Data Sheet
7 (1) The sale or importation of a controlled product whose chemical composition is similar to the chemical composition of other controlled products in its group is exempt from the application of paragraph 13(a) or 14(a) of the Act in respect of the requirement to transmit, obtain or prepare a material safety data sheet for the controlled product if a generic material safety data sheet for the group of controlled products is transmitted, obtained or prepared.
(2) The generic material safety data sheet referred to in subsection (1) shall disclose
(a) where the range of concentration of an ingredient of the controlled product is different from the range of concentration of the ingredient in the other controlled products in the group, beside the name of the controlled product and the ingredient, the range of concentration of the ingredient in the controlled product, in accordance with section 11; and
(b) where the hazard information in respect of the controlled product is different from the hazard information in respect of the other controlled products in the group, beside the name of the controlled product, the hazard information for the controlled product.
Employer Exemptions
8 The sale of a controlled product to an employer is exempt from the application of paragraph 13(a) of the Act in respect of the requirement to disclose information that could be the subject of a claim for exemption under subsection 11(2) of the Hazardous Materials Information Review Act if
(a) the employer has filed a claim for exemption or is exempt from disclosing that information in respect of the controlled product under
(i) the Hazardous Materials Information Review Act, or
(ii) the laws of a province; and
(b) the material safety data sheet of the controlled product transmitted in respect of that sale discloses in place of that information
(i) the information referred to in section 26 or 27, or
(ii) where the information referred to in section 26 or 27 is not available, an emergency telephone number of the employer that will enable a physician or nurse to obtain any information referred to in paragraph 13(a) of the Act that is in the possession of the employer for the purpose of making a medical diagnosis of, or rendering medical treatment to, a person in an emergency.
Secondary Supplier Exemptions
8.1 [Repealed, SOR/97-543, s. 14]
8.2 The sale of a controlled product by a supplier, in this section referred to as the secondary supplier, is exempt from the application of paragraph 13(a) of the Act in respect of the requirement to disclose on a material safety data sheet the chemical identity or concentration of an ingredient of the controlled product if
(a) the ingredient is sold either directly or indirectly by another supplier, in this section referred to as the primary supplier, who has filed a claim for exemption or is exempted from disclosing the chemical identity or concentration of the ingredient under the Hazardous Materials Information Review Act;
(b) the chemical identity or concentration of the ingredient is
(i) unknown to the secondary supplier, or
(ii) known to the secondary supplier and the secondary supplier has obtained the information in confidence, express or implied, and is obligated by contract or trust, express or implied, or otherwise by law or equity to maintain the confidentiality of the information;
(c) the material safety data sheet for the controlled product transmitted by the secondary supplier in respect of the sale discloses, in place of the chemical identity or concentration of the ingredient,
(i) the information referred to in section 26 or 27 in respect of
(A) where the secondary supplier has filed a claim for exemption or is exempted from disclosing information that could be used to identify the primary supplier, that claim or exemption, or
(B) in any other case, the primary supplier’s claim or exemption with the words “other supplier” in parentheses after that information,
(ii) where the primary supplier has filed a claim for exemption or is exempted from disclosing the chemical identity of the ingredient, the generic chemical identity of the ingredient as disclosed by the primary supplier, and
(iii) where the primary supplier has filed a claim for exemption or is exempted from disclosing the concentration of the ingredient, the concentration of the primary supplier’s controlled product in the secondary supplier’s controlled product; and
(d) the secondary supplier transmits with the material safety data sheet for the controlled product the material safety data sheet transmitted by the primary supplier in respect of the sale to the secondary supplier.
- SOR/88-555, s. 1
Laboratory Sample
9 (1) Where a supplier has not obtained or prepared a material safety data sheet in respect of a controlled product, the sale or importation of a laboratory sample of the controlled product is exempt from the application of paragraph 13(a) or 14(a) of the Act in respect of the requirement to transmit, obtain or prepare a material safety data sheet if the laboratory sample is packaged in a container that
(a) contains a quantity of less than 10 kilograms of the controlled product; and
(b) has a label applied to it in accordance with section 16.
(2) The sale or importation of a laboratory sample of a controlled product is exempt from the application of paragraph 13(a) or 14(a) of the Act in respect of the requirement to disclose the chemical identity of an ingredient of the controlled product on a material safety data sheet if
(a) the laboratory sample is intended solely to be tested for research and development purposes; and
(b) the generic chemical identity of the ingredient is disclosed on the material safety data sheet.
- SOR/2001-254, s. 1(F)
Laboratory Supply House
10 The sale or importation of a controlled product is exempt from the application of paragraph 13(a) or 14(a) of the Act in respect of the requirement to transmit, obtain or prepare a material safety data sheet if
(a) the controlled product
(i) originates from a laboratory supply house,
(ii) is intended for use in a laboratory, and
(iii) is packaged in a container in a quantity of less than 10 kilograms; and
(b) all the information required by paragraph 13(a) or 14(a) of the Act is disclosed on the label of the container in which the controlled product is packaged.
Mixtures containing Non-radioactive Carrier Materials
10.1 The sale or importation of a controlled product that is a mixture of one or more radioactive nuclides and one or more non-radioactive carrier materials is exempt from the application of paragraph 13(a) or 14(a) of the Act if
(a) the carrier material
(i) is present in an amount that is
(A) in the case of a liquid or gaseous carrier material, not more than 1.0 ml in volume, or
(B) in the case of a solid carrier material, not more than 1.0 g in weight, and
(ii) is not
(A) a carcinogen under Subdivision A of Division 2 of Class D referred to in section 54,
(B) a toxic or reactive material under Division 1 of Class 6 and Packing Group I of the Transportation of Dangerous Goods Regulations, or
(C) an infectious material under Division 3 of Class D of these Regulations or Division 2 of Class 6 of the Transportation of Dangerous Goods Regulations and can be handled in accordance with the physical containment requirements set out in Schedule I.1 to these Regulations;
(b) the carrier material is a vehicle for radioactive nuclides or radio-labelled compounds that are injected or ingested during medical or veterinary diagnostic or therapeutic procedures that have been approved by the Department of Health for routine clinical use; or
(c) the quantity of each radioactive nuclide is greater than the quantity specified for that radioactive nuclide in Part I of Schedule I to the Transport Packaging of Radioactive Materials Regulations.
- SOR/2001-254, s. 2
Range of Concentration of Ingredients
11 (1) For the purposes of this section, where the concentration of an ingredient of a controlled product or a complex mixture that is a component of a controlled product is expressed as a percentage, the percentage shall be an expression of the ratio of
(a) the weight of the ingredient or the complex mixture to the weight of the controlled product;
(b) the volume of the ingredient or the complex mixture to the volume of the controlled product; or
(c) the weight of the ingredient or the complex mixture to the volume of the controlled product.
(2) Where the concentration of an ingredient of a controlled product or a complex mixture that is a component of a controlled product is required to be disclosed on a material safety data sheet and the ingredient or complex mixture is not always present in the same concentration in the controlled product, the material safety data sheet may disclose, in lieu of the actual concentration of the ingredient or complex mixture, that the ingredient or complex mixture falls within one of the ranges of concentration set out in subsection (3), where the actual concentration of the ingredient or complex mixture falls within that range.
(3) For the purposes of subsection (2), the ranges of concentration are the following:
(a) from 0.1 to 1 per cent;
(b) from 0.5 to 1.5 per cent;
(c) from 1 to 5 per cent;
(d) from 3 to 7 per cent;
(e) from 5 to 10 per cent;
(f) from 7 to 13 per cent;
(g) from 10 to 30 per cent;
(h) from 15 to 40 per cent;
(i) from 30 to 60 per cent;
(j) from 40 to 70 per cent; and
(k) from 60 to 100 per cent.
(4) If the concentration of an ingredient in a controlled product is not always the same, a material safety data sheet may disclose, in lieu of the actual concentration of an ingredient of a controlled product or of a complex mixture that is a component of a controlled product, a range of concentration other than a range set out in subsection (3) if the disclosed range of concentration falls entirely within a range of concentration set out in subsection (3).
(5) Where the concentration of an ingredient of a controlled product or a complex mixture that is a component of a controlled product disclosed on a material safety data sheet is expressed as a percentage or in a range of concentration set out in subsection (3) or (4), the material safety data sheet shall also disclose which of the ratios set out in subsection (1) is being expressed.
- SOR/2001-254, s. 3
Information to be Disclosed on a Material Safety Data Sheet
12 (1) For the purposes of subparagraph 13(a)(v) and paragraph 14(a) of the Act, a material safety data sheet shall disclose the nine categories of information set out in Column I of an item of Schedule I and each category shall be identified by a heading that is the same as or similar to the suggested heading set out for that category in Column II of that item.
(2) Subject to subsections (3) to (10), for the purposes of subparagraph 13(a)(v) and paragraph 14(a) of the Act, the material safety data sheet of a controlled product shall disclose, under an appropriate heading set out in column II of an item of Schedule I or a similar heading, the information set out in each applicable subitem of column III of that item, including the unit of measure, where applicable, if the information is available to the supplier.
(3) The material safety data sheet of a controlled product shall disclose the information set out in column III of subitems 1(1) and 2(1) and (2) of Schedule I under the appropriate heading set out in column II of items 1 and 2 of that Schedule or the similar heading referred to in subsection (1).
(4) Information disclosed under one heading of a material safety data sheet is not required to be repeated under any other heading.
(5) Where any information required to be disclosed by subsection (2) is the subject of a claim for exemption or of an exemption under the Hazardous Materials Information Review Act, the information shall be replaced by the information required to be disclosed by section 26 or 27.
(6) Where there is no information disclosed in respect of a category set out in Column I of an item of Schedule I, the material safety data sheet shall disclose, under the heading for that category in English, the words “not available” or “not applicable”, as the case may be, and the material safety data sheet shall disclose, under the heading for that category in French, the words “pas disponible” or “sans objet”, as the case may be.
(7) Where a controlled product is sold to a distributor for the purpose of sale or resale, the distributor is not required, on the material safety data sheet, to disclose the supplier identifier of the distributor and particulars of the distributor, if the supplier identifier and particulars of the manufacturer or importer are disclosed on the material safety data sheet in accordance with subsection (2).
(8) Where a controlled product is packaged for a distributor by a manufacturer, the manufacturer is not required, on the material safety data sheet, to disclose the name of the manufacturer and particulars of the manufacturer, if the supplier identifier and particulars of the distributor are disclosed on the material safety data sheet in accordance with subsection (2).
(9) Where a supplier imports a controlled product for his own use, the supplier is not required, on the material safety data sheet, to disclose the name of the manufacturer and particulars of the manufacturer.
(10) Where the LD50 or LC50 of a controlled product that is a mixture is determined by testing the mixture, the supplier shall disclose, on the material safety data sheet for the controlled product, that LD50 or LC50 in place of the LD50 or LC50 of the ingredients of the mixture.
(11) A material safety data sheet shall disclose, in addition to the information required to be disclosed by subsection (2), any other hazard information with respect to the controlled product of which the supplier is aware or ought reasonably to be aware.
- SOR/97-543, s. 15
- SOR/2001-254, s. 4
13 (1) Where information respecting the toxicological properties of a controlled product disclosed on a material safety data sheet may be interpreted in such a way as to qualify or contradict other toxicological information disclosed on the material safety data sheet, the material safety data sheet shall include sufficient information concerning the toxicological studies so as not to mislead a person as to the nature or extent of the hazard posed by the controlled product.
(2) For the purposes of determining whether information may mislead a person as to the nature or extent of a hazard, the general impression that the information conveys shall be taken into account.
PART IILabels
Exemptions
Inner Containers
14 (1) For the purposes of this section, outer container means the most outward container of a controlled product that is visible under normal conditions of storage and handling, but does not include the most outward container if it is the only container of the controlled product. (contenant extérieur)
(2) The sale or importation of a controlled product is exempt from the application of paragraph 13(b) or 14(b) of the Act in respect of the requirement to apply a label to a container that is
(a) the inner container of the controlled product, if
(i) the outer container is not labelled in accordance with paragraph (d),
(ii) the person to whom the controlled product is sold undertakes in writing to apply a label to the inner container in accordance with paragraph 13(b) or 14(b) of the Act, and
(iii) in the case of a controlled product that is a mixture of one or more radioactive nuclides and one or more non-radioactive carrier materials, the mixture is packaged in more than one container and the outer container is labelled as required by these Regulations;
(b) a package liner of the controlled product;
(c) the outer container of the controlled product, if the label on an inner container is visible and legible through the outer container under normal conditions of storage and handling; or
(d) the outer container of a controlled product, if the outer container has applied to it a label in accordance with the Transportation of Dangerous Goods Regulations.
- SOR/88-555, s. 2
- SOR/2001-254, s. 5
Bulk Shipments
15 (1) The sale or importation of a bulk shipment of a controlled product is exempt from the application of paragraph 13(b) or 14(b) of the Act if
(a) a label, material safety data sheet or statement in writing disclosing the information required to be disclosed by section 19 in respect of the controlled product is transmitted to the person to whom the controlled product is sold on or before the date on which the person receives the bulk shipment; or
(b) the supplier has transmitted to the person to whom the controlled product is sold or the supplier who imports the controlled product has in his possession a label, material safety data sheet or statement in writing that
(i) is for a controlled product that has the same product identifier, and
(ii) discloses the information that is required to be disclosed by section 19 in respect of the controlled product and is current at the time of the sale or importation.
(2) For the purposes of subsection (1), where the information is transmitted on a material safety data sheet or a statement in writing, hazard symbols required to be disclosed by paragraph 19(1)(d) in respect of the controlled product may be replaced by reference to the class and, in the case of a controlled product included in Class D — Poisonous and Infectious Materials, the division into which the controlled product falls.
15.1 [Repealed, SOR/97-543, s. 16]
Laboratory Samples
16 Where a supplier has not obtained or prepared a material safety data sheet in respect of a controlled product, the sale or importation of a laboratory sample of the controlled product is exempt from the application of paragraph 13(b) or 14(b) of the Act if the laboratory sample is packaged in a container that
(a) contains a quantity of less than 10 kilograms of the controlled product; and
(b) has a label applied to it that discloses, in respect of the controlled product, the following information:
(i) the product identifier,
(ii) the chemical identity or generic chemical identity of any ingredient of the controlled product referred to in any of subparagraphs 13(a)(i) to (iv) of the Act, if known by the supplier,
(iii) the supplier identifier,
(iv) the statement “Hazardous Laboratory Sample. For hazard information or in an emergency, call (number disclosed under subparagraph (v))/Échantillon pour laboratoire de produit dangereux. Pour obtenir des renseignements sur les dangers ou en cas d’urgence, composer (le numéro divulgé en vertu du sous-alinéa (v))”, and
(v) an emergency telephone number of the supplier that will enable
(A) a user of the controlled product to obtain hazard information in respect of the controlled product, and
(B) a physician or nurse to obtain any information in respect of the controlled product that is referred to in paragraph 13(a) of the Act and is in the possession of the supplier for the purpose of making a medical diagnosis of, or rendering treatment to, a person in an emergency.
Laboratory Supply Houses
17 The sale or importation of a controlled product is exempt from the application of paragraph 13(b) or 14(b) of the Act if
(a) the controlled product
(i) originates from a laboratory supply house,
(ii) is intended for use in a laboratory, and
(iii) is packaged in a container in a quantity of less than 10 kilograms; and
(b) the container in which the controlled product is packaged has applied to it a label that discloses the following information in respect of the controlled product:
(i) the product identifier,
(ii) where a material safety data sheet is available, a statement to that effect,
(iii) risk phrases that are appropriate to the controlled product or to the classes, divisions or subdivisions into which the controlled product falls,
(iv) precautionary measures to be followed when handling, using or being exposed to the controlled product, and
(v) where appropriate, first aid measures to be taken in case of exposure to the controlled product.
Radioactive Nuclide Mixtures
17.1 The sale or importation of a controlled product that is a mixture of one or more radioactive nuclides and one or more non-radioactive carrier materials is exempt from the application of paragraph 13(b) or 14(b) of the Act if
(a) the carrier material
(i) is present in an amount that is
(A) in the case of a liquid or gaseous carrier material, no more than 1.0 ml in volume, or
(B) in the case of a solid carrier material, no more than 1.0 g in weight, and
(ii) is not
(A) a carcinogen under Subdivision A of Division 2 of Class D referred to in section 54,
(B) a toxic or reactive material under Division 1 of Class 6 and Packing Group I of the Transportation of Dangerous Goods Regulations, or
(C) an infectious material under Division 3 of Class D of these Regulations or Division 2 of Class 6 of theTransportation of Dangerous Goods Regulations and can be handled in accordance with the physical containment requirements set out in Schedule I.1 to these Regulations;
(b) the carrier material is a vehicle for radioactive nuclides or radio-labelled compounds that are injected or ingested during medical or veterinary diagnostic or theraputic procedures that have been approved by the Department of Health for routine clinical use; or
(c) the quantity of each radioactive nuclide is greater than the quantity specified for that radioactive nuclide in Part I of Schedule I to the Transport Packaging of Radioactive Materials Regulations.
- SOR/2001-254, s. 6
Labels of Bulk Shipments
18 For the purposes of subsection 11(2) of the Act, a label of a bulk shipment is included with or accompanies the bulk shipment when it is included with the shipping documents that accompany the bulk shipment.
Information to be Disclosed on Labels
19 (1) The label applied to a controlled product or the container in which a controlled product is packaged shall disclose, in respect of the controlled product, the following information:
(a) the product identifier;
(b) subject to subsections (3) and (4), the supplier identifier;
(c) a statement to the effect that a material safety data sheet is available;
(d) subject to subsection (5), hazard symbols set out in Column II of Schedule II that correspond with the classes in which the controlled product is included and the divisions into which the controlled product falls as set out in Column I of that Schedule; and
(e) where the container has a capacity of more than 100 millilitres, the following information:
(i) risk phrases that are appropriate to the controlled product or to the classes, divisions or subdivisions into which the controlled product falls,
(ii) precautionary measures to be followed when handling, using or being exposed to the controlled product, and
(iii) where appropriate, first aid measures to be taken in case of exposure to the controlled product.
(2) Paragraphs (1)(a) and (b) do not apply in respect of the sale of a controlled product to an employer who has filed a claim for exemption or is exempt under the Hazardous Materials Information Review Act or under the laws of a province from disclosing
(a) the chemical name, common name, generic name, trade name or brand name of a controlled product, if the label discloses the code name or code number specified by the supplier; or
(b) any information that could be used to identify the supplier of the controlled product, if that information is replaced by
(i) the information referred to in section 26 or 27, or
(ii) where the information referred to in section 26 or 27 is not available, the information required to be disclosed under the laws of the province.
(3) Where a controlled product is sold to a distributor for the purpose of sale or resale, the distributor is not required, in the information disclosed on the label under paragraph (1)(b), to disclose the supplier identifier of the distributor if the supplier identifier of the manufacturer or importer is disclosed on the label.
(4) Where a controlled product is packaged for a distributor by a manufacturer, the manufacturer is not required, in the information disclosed on the label under paragraph (1)(b), to disclose the supplier identifier of the manufacturer if the supplier identifier of the distributor is disclosed on the label.
(5) Where a controlled product falls into Divisions 1 and 2 of Class D — Poisonous and Infectious Material, paragraph (1)(d) does not apply in respect of the requirement to disclose on the label applied to the controlled product or the container in which the controlled product is packaged the hazard symbol set out in Column II of Schedule II that corresponds to Division 2 of Class D — Poisonous and Infectious Material as set out in Column I of that Schedule.
(6) Paragraphs (1)(b) and (e) do not apply to the sale or importation of a controlled product that is a mixture of one or more radioactive nuclides and one or more non-radioactive carrier materials.
- SOR/2001-254, s. 7
Label Design
20 (1) The label of a controlled product or container in which a controlled product is packaged shall be applied
(a) within a border that is
(i) in a colour that contrasts with the background against which it appears, and
(ii) designed as depicted in Schedule III; and
(b) on a part of the controlled product or container that is displayed under normal conditions of storage and use.
(2) Paragraph (1)(a) does not apply in respect of the sale or importation of a controlled product that is packaged in a container that meets the requirements of section 17.
- SOR/88-555, s. 4
Legibility of Labels
21 (1) The information required to be disclosed on the label of a controlled product or container in which a controlled product is packaged shall be clearly and prominently displayed, easily legible and contrasted with other information on the controlled product or container.
(2) A label applied to a controlled product or container in which a controlled product is packaged shall be sufficiently durable and resistant under normal conditions of transport, storage and use to remain attached and legible.
Reproduction of Hazard Symbols
22 Any hazard symbol required to be displayed on a label shall
(a) except with respect to size and colour, be an exact reproduction of that hazard symbol as depicted in Schedule II; and
(b) be displayed in a colour that is not likely to create confusion with a safety mark required by Part V of the Transportation of Dangerous Goods Regulations.
PART IIIGeneral
Exemption
23 (1) The importation of a controlled product that is to be labelled or repackaged in Canada is exempt from the application of section 14 of the Act in respect of the requirements to obtain or prepare a material safety data sheet with respect to the controlled product and to have a label applied to the controlled product or container in which the controlled product is packaged on the following conditions:
(a) subject to subsection (2), the supplier provides to an inspector in each province into which the controlled product is imported, on or before the date of importation, a statement indicating
(i) that he intends to import the controlled product,
(ii) the nature of the controlled product to be imported,
(iii) the address of the premises in the province at which the controlled product is to be labelled or repackaged, and
(iv) the provinces into which the controlled product is to be imported; and
(b) the supplier, if so requested by an inspector in a province into which the controlled product is imported, provides to the inspector
(i) a sample of the controlled product on or before the date of importation,
(ii) the proposed dates and places of importation, and
(iii) the approximate quantity of the controlled product to be imported.
(2) A statement provided in accordance with paragraph (1)(a) is valid in respect of the importation of that controlled product to those premises for a period not exceeding three years from the date the supplier provides the statement to the inspector.
(3) An importer who imports a controlled product in accordance with subsection (1) shall obtain or prepare a material safety data sheet in respect of the controlled product in accordance with the Act and these Regulations before the controlled product is used or sold.
(4) An importer who imports a controlled product in accordance with subsection (1) shall apply a label to the controlled product or to the container in which the controlled product is packaged in accordance with paragraph 14(b) of the Act
(a) where the controlled product is delivered to the address of the importer for his use or for sale, before the controlled product is used or sold; and
(b) where the controlled product is imported to the address of the person to whom the importer has sold the controlled product, before the controlled product is used by that person.
(5) Paragraph (4)(b) does not apply where the person to whom the importer sold the controlled product undertakes in writing to apply a label to the controlled product or to the container in which the controlled product is sold in accordance with paragraph 13(b) of the Act.
Manner of Disclosing Information
24 (1) The information required to be disclosed on a material safety data sheet of a controlled product shall be disclosed at the time of the sale of the controlled product in English and in French on a single material safety data sheet or on two material safety data sheets.
(2) Where a supplier transmits a material safety data sheet in respect of a controlled product, the information shall be disclosed on the material safety data sheet in English or in French, or both, in accordance with the request of the person to whom the controlled product is sold or, where the person does not specify the language in which the information shall be transmitted, the information shall be transmitted in English or in French, whichever is used in the course of the sale between the supplier and the person.
(3) The information required to be disclosed on the label of a controlled product or the container in which a controlled product is packaged shall be disclosed in English and in French.
25 The information required to be disclosed on a material safety data sheet, on a label of a controlled product or on a container in which a controlled product is packaged shall not be disclaimed or contradicted by information in respect of the controlled product that is
(a) not required under the Act; and
(b) disclosed on the material safety data sheet, the label, the controlled product or the container.
- SOR/97-543, s. 17
Information in Respect of Exemptions
26 (1) A supplier who, pursuant to subsection 11(1) of the Hazardous Materials Information Review Act, files a claim for exemption from a requirement to disclose information in respect of a controlled product on a material safety data sheet or on a label shall, in respect of the sale or importation of the controlled product or any controlled product having the same product identifier, disclose on the material safety data sheet and, where applicable, on the label of the controlled product or container in which the controlled product is packaged the date that the claim for exemption was filed and the registry number assigned to the claim under the Hazardous Materials Information Review Act.
(2) The requirements of subsection (1) apply in respect of a supplier who receives notice of a decision that the claim for exemption is valid
(a) if there is no appeal of the decision under subsection 20(1) of the Hazardous Materials Information Review Act, for a period not exceeding 30 days after the expiry of the appeal period; and
(b) if there is an appeal of the decision under subsection 20(1) of the Hazardous Materials Information Review Act, for a period not exceeding 30 days after the expiry of all periods for the making of an appeal or an application for judicial review in respect of the decision on appeal.
- SOR/2001-254, s. 8
- SOR/2004-317, s. 1
27 A supplier who receives notice of a decision made pursuant to the Hazardous Materials Information Review Act that his claim or a portion of his claim for exemption from a requirement to disclose information in respect of a controlled product on a material safety data sheet or a label is valid shall, during the period beginning not more than 30 days after the final disposition of the claim and ending on the last day of the exemption period, in respect of the sale or importation of the controlled product or any controlled product having the same product identifier, disclose on the material safety data sheet and, where applicable, on the label of the controlled product or container in which the controlled product is packaged the following information:
(a) a statement that an exemption has been granted;
(b) the date of the decision granting the exemption;
(c) the registry number assigned to the claim under the Hazardous Materials Information Review Act; and
(d) the generic chemical identity of the controlled product or ingredient as required by section 16 of the Act.
- SOR/88-555, s. 5
- SOR/97-543, s. 18
Identical Product Identifiers
28 The product identifier that is disclosed on the label of a controlled product or container in which a controlled product is packaged shall be identical to the product identifier that is disclosed on the material safety data sheet for the controlled product.
Revisions to Material Safety Data Sheets and Labels
29 (1) Where new information in respect of a controlled product or an ingredient of a controlled product becomes available, the supplier shall
(a) revise the material safety data sheet and the date thereof and, where applicable, the label of the controlled product; and
(b) in respect of the revised material safety data sheet and, where applicable, the revised label,
(i) in the case of the sale of the controlled product subsequent to the information becoming available, transmit the revised material safety data sheet and apply the revised label in accordance with section 13 of the Act, and
(ii) in the case of an importation of the controlled product subsequent to the information becoming available, obtain or prepare the revised material safety data sheet and apply the revised label in accordance with section 14 of the Act.
(2) Where no new information in respect of a controlled product or an ingredient of a controlled product becomes available in the three years following the date of preparation of a material safety data sheet of the controlled product, the supplier shall
(a) review the accuracy of the information disclosed on the material safety data sheet and, if necessary, revise the material safety data sheet and, where applicable, the label of the controlled product;
(b) revise the date of preparation disclosed on the material safety data sheet; and
(c) in respect of the revised material safety data sheet and, where applicable, the revised label of the controlled product,
(i) in the case of the sale of the controlled product after the revision of the material safety data sheet, transmit the revised material safety data sheet and, where applicable, apply the revised label in accordance with section 13 of the Act, and
(ii) in the case of an importation of the controlled product after the revision of the material safety data sheet, obtain or prepare the revised material safety data sheet and, where applicable, apply the revised label in accordance with section 14 of the Act.
Provision of Information
30 (1) Any supplier who sells or imports a controlled product intended for use in a work place in Canada shall provide, as soon as is practicable in the circumstances, any information in respect of the controlled product that is referred to in paragraph 13(a) of the Act and is in the possession of the supplier to any physician or nurse who requests that information for the purpose of making a medical diagnosis of, or rendering medical treatment to, a person in an emergency.
(2) Any physician or nurse to whom information is provided by a supplier pursuant to subsection (1) shall keep confidential any information specified by the supplier as being confidential except for the purpose for which it was provided.
31 Subject to the Hazardous Materials Information Review Act, a supplier who sells or imports a controlled product intended for use in a work place in Canada shall identify as soon as is practicable in the circumstances, on the request of an inspector, any person to whom a controlled product is sold or any user of a controlled product, the source of information for any toxicological data used in the preparation of any material safety data sheet that has been transmitted by the supplier to any person pursuant to paragraph 13(a) of the Act or has been obtained or prepared by the supplier pursuant to paragraph 14(a) of the Act.
PART IVClasses of Controlled Products
Interpretation
32 In this Part,
- ACGIH
ACGIH means the American Conference of Governmental Industrial Hygienists; (ACGIH)
- acute lethality
acute lethality means death of animals immediately or within 14 days after a single administration of or exposure to a toxic substance; (létalité aiguë)
- aerosol container
aerosol container means a disposable container designed to release pressurized contents by means of a manually operated valve which forms an integral part of the container; (contenant aérosol)
- ASTM
ASTM means the American Society for Testing and Materials; (ASTM)
- chronic toxic effect
chronic toxic effect means an adverse effect to the health of a person or test animal that develops
(a) over time, following a single exposure to a toxic substance, or
(b) from prolonged or repeated exposure to a toxic substance under conditions that do not produce that effect from a single exposure; (toxicité chronique)
- dust
dust means solid airborne particles that are mechanically generated; (poussières)
- flame projection
flame projection means the ignited discharge of the pressurized contents of an aerosol container; (projection de la flamme)
- flashback
flashback means that part of a flame projection that extends from its point of ignition back to the aerosol container; (retour de la flamme)
- flash point
flash point means the minimum temperature at which a liquid gives off vapour in sufficient concentration to ignite in test circumstances; (point d’éclair)
- fume
fume means solid particles in the air that are generated by condensation from the vapour of a solid material; (fumée)
- IARC
IARC means the International Agency for Research on Cancer; (CIRC)
- mist
mist means liquid droplets suspended in the air that are produced by the dispersion of a liquid or by the condensation of a vapourized liquid; (brouillard)
- NACE
NACE means the National Association of Corrosion Engineers (U.S.A.); (NACE)
- normal atmospheric pressure
normal atmospheric pressure means an absolute pressure of 101.325 kilopascals (1.00 atmosphere) at 20°C (68°F); (pression atmosphérique normale)
- OECD
OECD means the Organization for Economic Co-operation and Development; (OCDE)
- OECD Test Guideline
OECD Test Guideline means a test published in the OECD Standard entitled OECD Guidelines for Testing of Chemicals; (ligne directrice de l’OCDE)
- respiratory tract sensitization
respiratory tract sensitization means the development in a non-atopic person of severe asthma-like symptoms on exposure to a substance to which the person has previously been exposed; (sensibilisation des voies respiratoires)
- skin sensitization
skin sensitization means an immunologically-mediated, cutaneous reaction in a non-atopic person or animal on exposure to a substance to which the person or animal has previously been exposed; (sensibilisation de la peau)
- statistically significant
statistically significant means shown by statistical procedures to have a high probability of being due to something other than chance; (statistiquement significative)
- vapour
vapour means the gaseous form of a substance that is found in a solid or liquid state at normal atmospheric pressure. (vapeur)
- SOR/97-543, s. 19(E)
- SOR/2001-254, s. 9
Manner of Establishing Classification
33 (1) For the purpose of establishing that a product, material or substance is included in a class listed in Schedule II of the Act or falls into a division of a class, the supplier shall use, subject to subsection (2),
(a) results from testing that he has carried out with respect to the product, material or substance in accordance with sections 34 to 66, as applicable; or
(b) evaluation and scientific judgment based on test results with respect to
(i) the product, material or substance, or
(ii) where appropriate, a product, material or substance that has similar properties.
(2) For the purpose of establishing that a product, material or substance is or is not included in Class D — Poisonous and Infectious Material, the supplier may use information of which the supplier is aware or ought reasonably to be aware in place of the criteria set out in subsection (1).
(3) Where the test results referred to in paragraph (1)(b) are results from toxicological studies, the studies shall have been carried out in accordance with
(a) the applicable OECD Test Guideline referred to in this Part; or
(b) where there are no tests carried out in accordance with the applicable OECD Test Guidelines referred to in this Part, one of the following tests or methods:
(i) in the case of a 90 day test or a chronic test, a test or method described in U.S. Food and Drug Administration (FDA) guidelines or U.S. Environmental Protection Agency (EPA) guidelines, as published in the Federal Register and as amended from time to time,
(ii) in the case of a test for skin or eye irritation, the Draize Test as described in volume 82 of The Journal of Pharmacology and Experimental Therapeutics, dated 1944, at pages 377 to 390,
(iii) in the case of a test for teratogenicity, a test or method described in Principles for the Testing of Drugs for Teratogenicity, Technical Report Series Number 364, published in 1967 by the World Health Organization,
(iv) in the case of a test for mutagenicity, a test or method described by the U.S. Environmental Protection Agency (EPA) in “Proposed Guidelines for Registering Pesticides in the U.S.; Hazard Evaluation: Human and Domestic Animals”, as published in volume 43 of the Federal Register (No. 163), dated 1978, at pages 37,336 to 37,403, or
(v) any other test or method that is carried out in accordance with generally accepted standards of good scientific practice at the time the test is carried out.
- SOR/97-543, s. 20(E)
Class A — Compressed Gas
34 Any product, material or substance contained under pressure, including compressed gas, dissolved gas or gas liquefied by compression or refrigeration, that has any of the following characteristics shall be included in Class A — Compressed Gas listed in Schedule II to the Act:
(a) a critical temperature of less than 50°C (122°F);
(b) an absolute vapour pressure greater than 294 kilopascals (2.90 atmospheres) at 50°C (122°F);
(c) an absolute pressure in the cylinder or other pressure vessel in which it is packaged greater than 275±1 kilopascals (2.71±0.01 atmospheres) at 21.1°C (70°F) or 717±2 kilopascals (7.07±0.02 atmospheres) at 54.4°C (130°F); or
(d) in a liquid state, an absolute vapour pressure exceeding 275 kilopascals (2.71 atmospheres) at 37.8°C (100°F) as determined by the Standard Test Method for Vapor Pressure of Petroleum Products (Reid Method), ASTM D323-82, dated August 27, 1982.
- SOR/97-543, s. 21
Class B — Flammable and Combustible Material
35 (1) The products, materials and substances referred to in sections 36 to 41 shall be included in Class B — Flammable and Combustible Material listed in Schedule II to the Act.
(2) Divisions 1 to 6 are established as divisions of Class B — Flammable and Combustible Material listed in Schedule II to the Act.
Division 1: Flammable Gases
36 Any product, material or substance falls into Division 1 of Class B — Flammable and Combustible Material if it is a compressed gas included in Class A — Compressed Gas that, at normal atmospheric pressure forms a flammable mixture with air
(a) when in a concentration of 13 per cent or less by volume; or
(b) over a concentration range of at least 12 per cent by volume.
Division 2: Flammable Liquids
37 Any product, material or substance falls into Division 2 of Class B — Flammable and Combustible Material if it is a liquid that has a flash point of less than 37.8°C (100°F), when tested in accordance with the applicable method specified in Schedule IV for that type of liquid.
Division 3: Combustible Liquids
38 Any product, material or substance falls into Division 3 of Class B — Flammable and Combustible Material if it is a liquid that has a flash point of 37.8°C (100°F) or more but less than 93.3°C (200°F), when tested in accordance with the applicable method specified in Schedule IV for that type of liquid.
Division 4: Flammable Solids
39 Any product, material or substance falls into Division 4 of Class B — Flammable and Combustible Material if it is a solid that
(a) causes fire through friction or through retained heat from manufacturing or processing;
(b) can be ignited readily and when ignited burns so vigorously and persistently as to create a hazard;
(c) ignites readily and burns with a self-sustained flame at a rate of more than 0.254 centimetre (0.1 inch) per second along its major axis, when tested in accordance with the method set out in Schedule V; or
(d) is included in Division 1 of Class 4 of Part III of the Transportation of Dangerous Goods Regulations.
- SOR/97-543, s. 22(F)
Division 5: Flammable Aerosols
40 Any product, material or substance falls into Division 5 of Class B — Flammable and Combustible Material if it is packaged in an aerosol container and, when tested in accordance with the method set out in Schedule VI, yields a flame projection at full valve opening or a flashback at any degree of valve opening.
Division 6: Reactive Flammable Materials
41 Any product, material or substance falls into Division 6 of Class B — Flammable and Combustible Material if
(a) it is spontaneously combustible and liable to spontaneous heating under normal conditions of use or liable to heat in contact with air to the point where it begins to burn; or
(b) it emits a flammable gas or becomes spontaneously combustible on contact with water or water vapour.
Class C — Oxidizing Material
42 Any product, material or substance shall be included in Class C — Oxidizing Material listed in Schedule II to the Act if
(a) it causes or contributes to the combustion of another material by yielding oxygen or any other oxidizing substance, whether or not the product, material or substance is itself combustible; or
(b) it is an organic peroxide that contains the bivalent 0-0 structure.
Class D — Poisonous and Infectious Material
General
43 (1) The products, materials and substances referred to in sections 46 to 64 shall be included in Class D — Poisonous and Infectious Material listed in Schedule II to the Act.
(2) Divisions 1 to 3 are established as divisions of Class D — Poisonous and Infectious Material listed in Schedule II to the Act.
(3) Subdivisions A and B are established as subdivisions of Divisions 1 and 2 of Class D — Poisonous and Infectious Material listed in Schedule II to the Act.
(4) A gas included in Division 4 of Class 2 in Part III of the Transportation of Dangerous Goods Regulations does not fall into Division 1 or Division 2 of Class D — Poisonous and Infectious Material.
Formulae for Equivalent LC50
44 For the purpose of establishing that a product, material or substance falls into Division 1 of Class D — Poisonous and Infectious Material, an LC50 that is obtained in an animal assay at an exposure duration of other than four hours may be converted to an LC50 equivalent to an exposure duration of four hours by using the following formulae:
(a) for a gas or vapour,
LC50 at Y hours × (Y hours)½ ÷ 2 = LC50 at 4 hours; and
(b) for dust, mist or fume,
LC50 at Y hours × (Y hours) ÷ 4 = LC50 at 4 hours.
Note: Y = actual number of hours of exposure duration.
Toxicological Evaluation of Mixtures: LD50 and LC50 Data
45 (1) Subject to subsection (3), where the LD50 or LC50 of every ingredient of a mixture present at a concentration of one per cent or more is known, the LD50 or LC50 of the mixture shall be determined, taking into account all ingredients present at a concentration of one per cent or more, by using the following formulae:
(a) for a solid or a liquid,
(b) for a gas, vapour, dust, mist or fume,
Note: proportion = the weight of the ingredient divided by the weight of the mixture.
(2) Subject to subsection (3), where the LD50 or LC50 of one or more ingredients of a mixture is not known, the LD50 or LC50 of the mixture is equal to the LD50 or LC50 of the most acutely lethal ingredient that is present in the mixture at a concentration of one per cent or more and for which LD50 or LC50 data is available.
(3) The LD50 or LC50 of a mixture may be determined by testing the mixture.
DIVISION 1: Materials Causing Immediate and Serious Toxic EffectsSubdivision A: Very Toxic Material
Pure Substances and Tested MixturesAcute Lethality
46 A pure substance or tested mixture falls into Subdivision A of Division 1 of Class D — Poisonous and Infectious Material if, in an animal assay for acute lethality, it has an
(a) LD50 not exceeding 50 milligrams per kilogram of body weight of the animal when tested in accordance with OECD Test Guideline No. 401, “Acute Oral Toxicity”, dated May 12, 1981;
(b) LD50 not exceeding 200 milligrams per kilogram of body weight of the animal when tested in accordance with OECD Test Guideline No. 402, “Acute Dermal Toxicity”, dated May 12, 1981;
(c) LC50 not exceeding 2,500 parts per million by volume of gas when tested for four hours in accordance with OECD Test Guideline No. 403, “Acute Inhalation Toxicity”, dated May 12, 1981;
(d) LC50 not exceeding 1,500 parts per million by volume of vapour when tested for four hours in accordance with OECD Test Guideline No. 403, “Acute Inhalation Toxicity”, dated May 12, 1981, and a saturated vapour concentration at normal atmospheric pressure greater than two times the value of that LC50; or
(e) LC50 not exceeding 0.5 milligrams per litre or 500 milligrams per cubic metre of dust, mist or fume when tested for four hours in accordance with OECD Test Guideline No. 403, “Acute Inhalation Toxicity”, dated May 12, 1981.
Poisonous Substances as Defined by the Transportation of Dangerous Goods Regulations
47 A pure substance or tested mixture falls into Subdivision A of Division 1 of Class D — Poisonous and Infectious Material if it is included in Division 3 of Class 2 or in Packing Group I or II of Division 1 of Class 6 in Part III of the Transportation of Dangerous Goods Regulations.
Untested Mixtures
48 An untested mixture falls into Subdivision A of Division 1 of Class D — Poisonous and Infectious Material if it contains a product, material or substance that meets any of the criteria applicable to a pure substance or tested mixture referred to in section 46 or 47 and is present at a concentration of one per cent or more.
Subdivision B: Toxic Material
Pure Substances and Tested MixturesAcute Lethality
49 A pure substance or tested mixture falls into Subdivision B of Division 1 of Class D — Poisonous and Infectious Material if, in an animal assay for acute lethality, it has an
(a) LD50 of more than 50 but not exceeding 500 milligrams per kilogram of body weight of the animal, when tested in accordance with OECD Test Guideline No. 401, “Acute Oral Toxicity”, dated May 12, 1981;
(b) LD50 of more than 200 but not exceeding 1 000 milligrams per kilogram of body weight of the animal, when tested in accordance with OECD Test Guideline No. 402, “Acute Dermal Toxicity”, dated May 12, 1981;
(c) LC50 of more than 1,500 but not exceeding 2,500 parts per million by volume of vapour, when tested for four hours in accordance with OECD Test Guideline No. 403, “Acute Inhalation Toxicity”, dated May 12, 1981, and a saturated vapour concentration at normal atmospheric pressure of more than 0.4 times the LC50; or
(d) LC50 of more than 0.5 but not exceeding 2.5 milligrams per litre or grams per cubic metre of dust, mist or fume, when tested for four hours in accordance with OECD Test Guideline No. 403, “Acute Inhalation Toxicity”, dated May 12, 1981.
Poisonous Substances as Defined by the Transportation of Dangerous Goods Regulations
50 A pure substance or tested mixture falls into Subdivision B of Division 1 of Class D — Poisonous and Infectious Material if it is included in Packing Group III of Division 1 of Class 6 in Part III of the Transportation of Dangerous Goods Regulations.
Untested Mixtures
51 An untested mixture falls into Subdivision B of Division 1 of Class D — Poisonous and Infectious Material if it contains a product, material or substance that meets any of the criteria applicable to a pure substance or tested mixture referred to in section 49 or 50 and is present at a concentration of one per cent or more.
DIVISION 2: Materials Causing Other Toxic EffectsSubdivision A: Very Toxic Material
Pure Substances and Tested MixturesChronic Toxic Effects
52 A pure substance or tested mixture falls into Subdivision A of Division 2 of Class D — Poisonous and Infectious Material if, in an animal assay for chronic toxic effects, it elicits a response of sufficient severity to threaten life or cause serious permanent impairment in a statistically significant proportion of the test population at
(a) a dose not exceeding 10 milligrams per kilogram of body weight of the animal per day when tested in accordance with
(i) OECD Test Guideline No. 408, “Subchronic Oral Toxicity — Rodent: 90-day”, dated May 12, 1981,
(ii) OECD Test Guideline No. 409, “Subchronic Oral Toxicity — Non-Rodent: 90-day”, dated May 12, 1981, or
(iii) the oral route test in OECD Test Guideline No. 452, “Chronic Toxicity Studies”, dated May 12, 1981;
(b) a dose not exceeding 20 milligrams per kilogram of body weight of the animal per day when tested in accordance with
(i) OECD Test Guideline No. 411, “Subchronic Dermal Toxicity: 90-day”, dated May 12, 1981, or
(ii) the dermal route test in OECD Test Guideline No. 452, “Chronic Toxicity Studies”, dated May 12, 1981; or
(c) a concentration not exceeding 25 parts per million by volume of gas or vapour, or not exceeding 10 micrograms per litre or 10 milligrams per cubic metre of dust, mist or fume when tested in accordance with
(i) OECD Test Guideline No. 413, “Subchronic Inhalation Toxicity: 90-day”, dated May 12, 1981, or
(ii) the inhalation route test in OECD Test Guideline No. 452, “Chronic Toxicity Studies”, dated May 12, 1981.
Teratogenicity and Embryotoxicity
53 (1) A pure substance or tested mixture falls into Subdivision A of Division 2 of Class D — Poisonous and Infectious Material if, in an animal assay for teratogenicity and embryotoxicity, it is shown to cause injury to the embryo or fetus in a statistically significant proportion of the test population at a concentration that has no adverse effect on the pregnant female when tested in accordance with
(a) OECD Test Guideline No. 414, “Teratogenicity”, dated May 12, 1981;
(b) OECD Test Guideline No. 415, “One-Generation Reproduction Toxicity”, dated May 26, 1983; or
(c) OECD Test Guideline No. 416, “Two-Generation Reproduction Toxicity”, dated May 26, 1983.
(2) In this section, injury includes death, malformation, permanent metabolic or physiological disfunction, growth retardation or psychological or behavioural alteration that occurs during pregnancy, at birth or in the postnatal period.
Carcinogenicity
54 A pure substance or tested mixture falls into Subdivision A of Division 2 of Class D — Poisonous and Infectious Material if it is listed in
(a) section Ala, Alb or A2 of Appendix A of the Threshold Limit Values for Chemical Substances and Physical Agents in the Work Environment, published by the ACGIH, as amended from time to time; or
(b) Group 1 or Group 2 in the IARC Monographs on the Evaluation of the Carcinogenic Risk of Chemicals to Humans, published by the World Health Organization, as amended from time to time.
Reproductive Toxicity
55 A pure substance or tested mixture falls into Subdivision A of Division 2 of Class D — Poisonous and Infectious Material if
(a) there is evidence that shows that it causes sterility or an adverse effect on reproductive capability in persons following exposure to it in the work place; or
(b) sterility or an adverse effect on reproductive capability is shown in an animal assay for reproductive toxicity carried out in accordance with
(i) OECD Test Guideline No. 415, “One-Generation Reproduction Toxicity”, dated May 26, 1983, or
(ii) OECD Test Guideline No. 416, “Two-Generation Reproduction Toxicity”, dated May 26, 1983.
Respiratory Tract Sensitization
56 A pure substance or tested mixture falls into Subdivision A of Division 2 of Class D — Poisonous and Infectious Material if there is evidence that shows that it causes respiratory tract sensitization in persons following exposure to it in the work place.
Mutagenicity
57 (1) A pure substance or tested mixture falls into Subdivision A of Division 2 of Class D — Poisonous and Infectious Material if
(a) there is epidemiological evidence that shows a causal connection between exposure of persons to the substance or mixture and heritable genetic effects; or
(b) there is evidence of mutagenicity in mammalian germ cells in vivo as shown by
(i) positive results in a study that measures mutations transmitted to offspring, or
(ii) positive results in an in vivo study showing chemical interaction with the genetic materials of mammalian germ cells and positive results in an in vivo study assessing either gene mutation or chromosomal aberration in somatic cells.
(2) The evidence referred to in paragraph (1)(b) shall be obtained
(a) in accordance with test methods described in the “Introduction to the OECD Guidelines on Genetic Toxicology Testing and Guidance on the Selection and Application of Assays”, dated March 1, 1987, published in the Third Addendum to the OECD Guidelines for Testing of Chemicals; and
(b) using testing strategies described in the Guidelines on the Use of Mutagenicity Tests in the Toxicological Evaluation of Chemicals, dated 1986, published under the authority of the Minister of National Health and Welfare and the Minister of the Environment.
- SOR/97-543, s. 23(F)
Untested Mixtures
58 An untested mixture falls into Subdivision A of Division 2 of Class D — Poisonous and Infectious Material if it contains a product, material or substance that meets the criteria applicable to a pure substance or tested mixture referred to in
(a) any of sections 53 to 57, if the product, material or substance is present at a concentration of 0.1 per cent or more; or
(b) section 52, if the product, material or substance is present at a concentration of one per cent or more.
Subdivision B: Toxic Material
Pure Substances and Tested MixturesChronic Toxic Effects
59 A pure substance or tested mixture falls into Subdivision B of Division 2 of Class D — Poisonous and Infectious Material if, in an animal assay for chronic toxic effects, it elicits a response of sufficient severity to threaten life or cause serious permanent impairment in a statistically significant proportion of the test population at
(a) a dose of more than 10 but not exceeding 100 milligrams per kilogram of body weight of the animal per day, when tested in accordance with
(i) OECD Test Guideline No. 408, “Subchronic Oral Toxicity — Rodent: 90-day”, dated May 12, 1981,
(ii) OECD Test Guideline No. 409, “Subchronic Oral Toxicity — Non-Rodent: 90-day”, dated May 12, 1981, or
(iii) the oral route test in OECD Test Guideline No. 452, “Chronic Toxicity Studies”, dated May 12, 1981;
(b) a dose of more than 20 but not exceeding 200 milligrams per kilogram of body weight of the animal per day, when tested in accordance with
(i) OECD Test Guideline No. 411, “Subchronic Dermal Toxicity: 90-day”, dated May 12, 1981, or
(ii) the dermal route test in OECD Test Guideline No. 452, “Chronic Toxicity Studies”, dated May 12, 1981; or
(c) a concentration of more than 25 but not exceeding 250 parts per million by volume of gas or vapour, or more than 10 but not exceeding 100 micrograms per litre or more than 10 but not exceeding 100 milligrams per cubic metre, of dust, mist or fume, when tested in accordance with
(i) OECD Test Guideline No. 413, “Subchronic Inhalation Toxicity: 90-day”, dated May 12, 1981, or
(ii) the inhalation route test in OECD Test Guideline No. 452, “Chronic Toxicity Studies”, dated May 12, 1981.
Skin or Eye Irritation
60 A pure substance or tested mixture falls into Subdivision B of Division 2 of Class D — Poisonous and Infectious Material if, in an animal assay,
(a) it causes an effect graded at a mean of two or more for erythema formation or two or more for edema formation, when tested in accordance with OECD Test Guideline No. 404, “Acute Dermal Irritation/Corrosion”, dated May 12, 1981, as measured at any of the times specified in the test; or
(b) it causes an effect graded at a mean of two or more for corneal damage, one or more for iris damage or 2.5 or more for conjunctival swelling or redness, when tested in accordance with OECD Test Guideline No. 405, “Acute Eye Irritation/Corrosion”, dated May 12, 1981, as measured at any of the times specified in the test.
- SOR/97-543, s. 24(F)
Skin Sensitization
61 A pure substance or tested mixture falls into Subdivision B of Division 2 of Class D — Poisonous and Infectious Material if
(a) in an animal assay carried out in accordance with OECD Test Guideline No. 406, “Skin Sensitization”, dated May 12, 1981,
(i) it produces a response in 30 per cent or more of the test animals, when using one of the techniques incorporating the use of an adjuvant, or
(ii) it produces a response in 15 per cent or more of the test animals, when using one of the techniques not incorporating the use of an adjuvant; or
(b) evidence shows that it causes skin sensitization in persons following exposure in a work place.
Mutagenicity
62 A pure substance or tested mixture falls into Subdivision B of Division 2 of Class D — Poisonous and Infectious Material if evidence of mutagenicity in mammalian somatic cells in vivo is obtained in a test to assess either gene mutation or chromosomal aberration carried out
(a) in accordance with test methods described in the “Introduction to the OECD Guidelines on Genetic Toxicology Testing and Guidance on the Selection and Application of Assays” published in the Third Addendum to the OECD Guidelines for Testing of Chemicals, dated March 1, 1987; and
(b) using testing strategies described in the Guidelines on the Use of Mutagenicity Tests in the Toxicological Evaluation of Chemicals, dated 1986, published by authority of the Minister of Health and the Minister of the Environment.
- SOR/97-543, s. 25
Untested Mixtures
63 An untested mixture falls into Subdivision B of Division 2 of Class D — Poisonous and Infectious Material if it contains a product, material or substance that meets any of the criteria applicable to a pure substance or tested mixture referred to in any of sections 59 to 62 and is present at a concentration of one per cent or more.
DIVISION 3: Biohazardous Infectious Material
64 An organism that has been shown to cause disease or is reasonably believed to cause disease in persons or animals and the toxins of such an organism fall into Division 3 of Class D — Poisonous and Infectious Material.
Class E — Corrosive Material
65 A product, material or substance shall be included in Class E — Corrosive Material listed in Schedule II to the Act if
(a) it corrodes SAE 1020 steel or 7075-T6 non-clad aluminum surfaces at a rate exceeding 6.25 millimetres per year at a test temperature of 55°C when tested in accordance with Test Method, Laboratory Corrosion Testing of Metals for the Process Industries, NACE Standard TM-01-69 (1976 Revision);
(b) it is corrosive to skin when tested in accordance with OECD Test Guideline No. 404, “Acute Dermal Irritation/Corrosion”, dated May 12, 1981;
(c) it is included in Class 8 in Part III of the Transportation of Dangerous Goods Regulations;
(d) it is a gas included in Division 4 of Class 2 in Part III of the Transportation of Dangerous Goods Regulations;
(e) there is evidence that it causes visible necrosis of human skin tissue; or
(f) it is an untested mixture containing a product, material or substance that meets the criteria referred to in paragraph (b) or (e) and is present at a concentration of at least one per cent.
Class F — Dangerously Reactive Material
66 A product, material or substance shall be included in Class F — Dangerously Reactive Material listed in Schedule II to the Act if it
(a) undergoes vigorous polymerization, decomposition or condensation;
(b) becomes self-reactive under conditions of shock or increase in pressure or temperature; or
(c) reacts vigorously with water to release a gas that has an LC50 not exceeding 2,500 parts per million by volume of gas, when tested for four hours in accordance with OECD Test Guideline No. 403, “Acute Inhalation Toxicity”, dated May 12, 1981.
SCHEDULE I(Section 12)
INFORMATION TO BE DISCLOSED ON A MATERIAL SAFETY DATA SHEET
| Column I | Column II | Column III | |
|---|---|---|---|
| Item | Category | Suggested Headings | Information in respect of controlled products |
| 1 | Hazardous Ingredients | Hazardous Ingredients |
|
| 2 | Preparation Information | Preparation Information |
|
| 3 | Product Information | Product Information |
|
| 4 | Physical Data | Physical Data |
|
| 5 | Fire or Explosion Hazard | Fire or Explosion Hazard |
|
| 6 | Reactivity Data | Reactivity Data |
|
| 7 | Toxicological Properties | Toxicological Properties |
|
| 8 | Preventive Measures | Preventive Measures |
|
| 9 | First Aid Measures | First Aid Measures |
|
- SOR/97-543, ss. 26(F), 27(E)
SCHEDULE I.1(Sections 10.1 and 17.1)Physical Containment Requirements for Low Individual or Community Risk Agents
1 In this Schedule, low individual or community risk agents includes microorganisms, bacteria, fungi, viruses and parasites that are unlikely to cause disease in healthy persons or animals.
2 The physical requirements for a basic laboratory for handling low individual or community risk agents are those of a well-designed and functional laboratory and, in particular, include the following:
(a) the room in which the laboratory is located must be separated from any public area by a door;
(b) if the room opens onto a public area or a heavily travelled corridor, the door to the room in which the laboratory is located must remain closed except when being used to enter or leave the laboratory;
(c) the finished surface of the walls, ceiling, furniture and floors of the laboratory must be washable;
(d) all working areas and containment equipment must be located away from windows that may be opened and that must be equipped with fly screens;
(e) handwashing facilities must be provided, preferably at a location near the exit to the corridor or to any public area; and
(f) laboratory coats must be hung in a separate area from the place where street clothing is hung.
- SOR/2001-254, s. 10
- SOR/2004-317, s. 2(F)
SCHEDULE II/ANNEXE II(Paragraphs 19(1)(d) and 22(a)/alinéas 19(1)d) et 22a))Hazard Symbols/Signaux de danger
| Column I | Column II / Colonne II | Colonne I |
|---|---|---|
| Classes and Divisions | Hazard Symbols / Signaux de danger | Catégories et Divisions |
| Class A – Compressed Gas |  | Catégorie A – Gaz comprimés |
| Class B – Flammable and Combustible Material |  | Catégorie B – Matières inflammables et combustibles |
| Class C – Oxidizing Material |  | Catégorie C – Matières comburantes |
| Class D – Poisonous and Infectious Material | Catégorie D – Matières toxiques et infectieuses | |
|  |
|
|  |
|
|  |
|
| Class E – Corrosive Material |  | Catégorie E – Matières corrosives |
| Class F – Dangerously Reactive Material |  | Catégorie F – Matières dangereusement réactives |
SCHEDULE III/ANNEXE III(Subparagraph 20(a)(ii)/sous-alinéa 20a)(ii))Label Border/Bordure d’étiquette
SCHEDULE IV(Sections 37 and 38)Methods of Testing for Flash Point
1 The method of testing for the flash point of
(a) a liquid, other than a liquid referred to in paragraph (c) or (d), having a viscosity of less than 5.8 mm2/s (45 Saybolt Universal Seconds) at 37.8°C (100°F) is the Standard Test Method for Flash Point by Tag Closed Tester ASTM D56-82, dated August 27, 1982, or the appropriate test in the Standard Test Methods for Flash Point by Setaflash Closed Tester, ASTM D3828-81, dated August 28, 1981;
(b) a liquid, other than a liquid referred to in paragraph (c) or (d), having a viscosity of 5.8 mm2/s (45 Saybolt Universal Seconds) or more at 37.8°C (100°F), is the appropriate test in the Standard Test Methods for Flash Point by Pensky-Martens Closed Tester, ASTM D93-80, dated August 29, 1980;
(c) an aviation turbine fuel is the appropriate test in the Standard Test Methods for Flash Point by Setaflash Closed Tester, ASTM D3828-81, dated August 28, 1981; and
(d) a paint, enamel, lacquer, varnish or similar liquid that has a flash point between 0°C (32°F) and 110°C (230°F) and a viscosity of less than 15 000 mm2/s at 25°C (77°F) determined in accordance with the Standard Test Method for Kinematic Viscosity of Transparent and Opaque Liquids (and the Calculation of Dynamic Viscosity), ASTM D445-83, dated October 28, 1983, is the appropriate test in the Standard Test Methods for Flash Point of Liquids by Setaflash Closed-Cup Apparatus, ASTM D3278-82, dated October 29, 1982.
- SOR/97-543, s. 28
SCHEDULE V(Paragraph 39(c))Method of Testing for Determining Flammable Solids That Ignite Readily
Preparation of Samples
1 For granules, powders and pastes, pack the sample into a flat, rectangular metal boat with inner dimensions 2.54 cm (1 inch) wide, 15.25 cm (6 inches) long and 0.63 cm (0.25 inch) deep.
2 For rigid and pliable solids, measure the dimensions of the sample and support it by means of metal ringstands, clamps, rings or other suitable devices as needed, so that the major axis is oriented horizontally and the maximum surface is freely exposed to the atmosphere.
Procedure
3 (1) Place the prepared sample in a draft-free area that can be ventilated and cleared after each test.
(2) The temperature of the sample at the time of testing shall be between 19.8°C (68°F) and 29.7°C (86°F).
(3) Hold a burning paraffin candle whose diameter is at least 2.54 cm (1 in.), so that the flame is in contact with the surface of the sample at the end of its major axis for five seconds or until the sample ignites, whichever is less, and remove the candle.
(4) Using a stopwatch, determine the length of time of combustion with self-sustained flame (the time not to exceed 60 seconds).
(5) Extinguish the flame with a C02 or similar non-destructive-type fire extinguisher.
(6) Measure the dimensions of the burnt area and calculate the rate of burning along the major axis of the sample in centimetres or inches per second.
- SOR/97-543, ss. 29, 30(F)
SCHEDULE VI(Section 40)Test for Determining the Flashback and the Length of the Flame Projection of Products, Materials and Substances Packaged in Aerosol Containers
Application
1 This test is for use in determining any flashback and the length of the flame projection of any product, material or substance packaged in an aerosol container.
Apparatus
2 The following apparatus shall be used in carrying out this test:
(a) a flammability tester, illustrated in the figure, that
(i) is designed so that the aerosol container can be secured in place by means of a device, such as a three-pronged clamp affixed to a ring stand, in such a manner that the discharge from the container is in the horizontal plane,
(ii) may include a device by which the valve of any aerosol container can be activated by remote control such as a side-pull, caliper-type bicycle hand brake,
(iii) has a vertically mounted burner
(A) that has an inside diameter of 1.2 mm,
(B) that has been made from a Luer-Lock 16 gauge needle affixed to a metal tube or from other suitable material or devices, and
(C) that is placed at a distance of 15 cm from the discharge orifice of the aerosol container, such distance to be measured horizontally between the vertical planes of the discharge orifice and the burner orifice, and
(iv) has two support frameworks
(A) each having an internal open space 35 cm wide by 45 cm high, constructed from metal or other non-flammable material and mounted in a vertical plane perpendicular to the direction of discharge from the aerosol container, one being at a distance of 15 cm from the burner and the other at a distance of 45 cm from the burner and both being on the opposite side of the burner from the container, and
(B) that are adjustable in the vertical plane;
(b) an n-butane gas cylinder (C.P. grade) fitted with a regulator capable of delivering pressure to the burner appropriate to maintaining the flame heights specified in subsection 4(5); and
(c) loosely woven cotton fabric commonly referred to as cheesecloth that has, in the bleached state, a mass per unit area of not less than 35 g/m2 and not more than 65 g/m2.
Test Specimen
3 (1) Where there are instructions by the manufacturer respecting the shaking of the aerosol container, a test comprised of three discharges from each of three aerosol containers of the same product and of the same size shall be conducted.
(2) Where there are no instructions by the manufacturer respecting the shaking of the aerosol container, a test comprised of three discharges from the container without shaking and subsequently, three discharges from the container after shaking, in accordance with subsection 4(9), from each of three aerosol containers of the same product and of the same size shall be conducted.
Procedure
4 (1) A test
(a) shall be carried out at a temperature of (22 ± 2) °C in the absence of air currents with an allowance made for a clearance of 50 cm beyond the framework set at a distance of 45 cm from the burner; and
(b) may be conducted in a fume hood with the exhaust fan turned off and the protecting door lowered.
(2) All the fumes shall be exhausted and the residues cleaned up after each discharge.
(3) Each aerosol container shall be conditioned to a temperature of (22 ± 2) °C and a discharge shall be released for five seconds from each aerosol container prior to testing.
(4) Install the first aerosol container in the device and ensure that the burner orifice is 15 cm from the discharge orifice in the horizontal plane and 5 cm below it in the vertical plane and that the discharge orifice points in the direction of the burner.
(5) Adjust the burner to give a flame height of 5 cm and release a trial discharge from the aerosol container and, if no flame projection occurs, lower the burner orifice by 5 cm and adjust the burner to give a flame height of 12 cm.
(6) Attach the cheesecloth to the flammability tester with bulldog clips or in any other manner so as to cover the entire internal space of the support framework set at a distance of 15 cm from the burner.
(7) Verify that the cheesecloth is at a proper horizontal distance from the vertical plane of the burner orifice (on the opposite side of the burner from the aerosol container).
(8) Adjust the height of the framework so that the cheesecloth will intercept the line of flame projection.
(9) Prepare the aerosol container in accordance with the manufacturer’s instructions and
(a) if shaking is applicable,
(i) shake vigorously for five seconds, or for the period of time specified in the manufacturer’s instructions,
(ii) install the container in the device, and
(iii) 15 seconds after the cessation of shaking, release the first discharge in accordance with subsection (10); or
(b) if shaking is not applicable, install the container in the device and release the discharge in accordance with subsection (10).
(10) Release a discharge
(a) until the valve of the aerosol container has been open for five seconds; or
(b) where any part of the cheesecloth ignites before the end of five seconds, until the time of such ignition.
(11) For each subsequent discharge of each aerosol container tested, allow the container to stand for at least 60 seconds, and
(a) if shaking is applicable, repeat the procedure referred to in paragraph (9)(a); or
(b) if shaking is not applicable, release the discharge in accordance with subsection (10).
(12) Where the cheesecloth mounted on the support framework set at a distance of 15 cm from the burner ignites, the remaining discharges referred to in section 3 shall be carried out in accordance with subsections (1) to (11), but with a new piece of cheesecloth attached to the support framework set at a distance of 45 cm from the burner.
Determination and Reporting of Flame Projection and Flashback
5 (1) Where, at any time during the test, the cheesecloth mounted at a distance of 45 cm from the burner in accordance with subsection 4(12) is ignited, the length of the flame projection is 45 cm or more.
(2) Where, at any time during the test, the cheesecloth mounted at a distance of 15 cm from the burner in accordance with subsection 4(6) is ignited but the cheesecloth mounted at a distance of 45 cm from the burner in accordance with subsection 4(12) is not ignited, the length of the flame projection is 15 cm or more but less than 45 cm.
(3) Where, at any time during the test, the cheesecloth mounted at a distance of 15 cm from the burner in accordance with subsection 4(6) is not ignited, but there is a flame projection, the length of the flame projection is less than 15 cm.
6 The following results shall be reported:
(a) the length of the flame projection;
(b) a lack of flame projection resulting from any of the test discharges; and
(c) any flashback.
FIGURE
Flammability Tester/Dispositif vérificateur d’inflammabilité
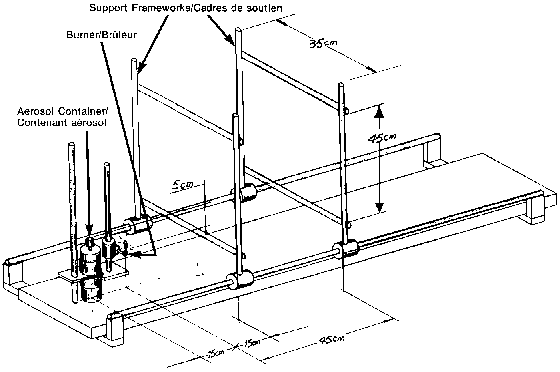
- Date modified: